Yuanda Pharmaceutical (00512) has a landmark breakthrough in multiple sectors: deepening the closed loop of “integration of diagnosis and treatment” of nuclear drugs, and multiple sectors have blossomed to create an innovative growth pole
Currently, the global nuclear drug anti-tumor diagnosis and treatment field is moving towards an era of “integrated diagnosis and treatment” at an unprecedented speed.
As one of only four pharmaceutical companies in the world that have successfully commercialized innovative nuclear drugs globally, Yuanda Pharmaceutical (00512) has recently achieved successive milestones in the anti-tumor diagnosis and treatment sector of nuclear drugs, marking a further acceleration of its global commercial layout:
GPN01530, a major global innovative radionuclide conjugation drug independently developed by the company, was recently approved by the US FDA to carry out phase I/II clinical studies to diagnose solid tumors, enabling the self-developed product to “go overseas.” At the same time, the TLx591-cdx phase III clinical trial for prostate cancer diagnosis in China also achieved positive top-line results and successfully reached major clinical endpoints.
In addition to its absolute advantage in the field of nuclear medicine, there are also frequent reports of Yuanda Pharmaceutical's differentiated innovation and development in various fields of advantage:
Neffy® (Neffy®), the world's first adrenaline nasal spray for emergency treatment of type I allergic reactions, was recently approved and marketed in China, filling the gap in the domestic out-of-hospital first aid market; in addition, the domestic phase II clinical study of the company's Class 1.1 innovative drug GPN01360 successfully reached a clinical end, showing significant efficacy and safety advantages in the treatment of depression, providing a “Chinese solution” for the treatment of depression.
This series of breakthroughs not only verified Yuanda Pharmaceutical's first-mover advantage on multiple tracks, but also revealed its deeper core competitiveness under the global innovation and development of “Go Global” represented by the nuclear drug sector.
In the nuclear medicine sector, where the company's global advantage is most prominent, Yuanda Pharmaceutical relies on “independent development+global expansion” two-wheel drive and has built a closed-loop platform for the entire industry chain covering research, production, sales and regulatory qualifications. From the commissioning of the Chengdu Intelligent Nuclear Drug Plant to the launch of the “China and US Double Report” model for the core pipeline, Yuanda Pharmaceutical is steadily being promoted to a leading global nuclear drug company, continuing to polish the golden business card of “Made in China” in the field of international medicine.
TLx591-cdx: Integrated diagnosis accelerates, overseas performance explodes
Yuanda Pharmaceutical's in-depth layout in the field of prostate cancer diagnosis and treatment is entering an outbreak period with the success of TLx591-cdx phase III clinical research in China.
The Zhitong Finance App learned that TLx591-cdx (Illuccix®, gallium Ga 68 PSMA-11) is a diagnostic RDC drug targeting prostate-specific membrane antigen (PSMA). Its phase III clinical trial in China achieved positive top-line results, and the main clinical endpoints were successfully achieved.
Clinical results showed that the total positive predictive value (PPV) of TLx591-CDx tumors was extremely high, reaching 94.8% (confidence interval, CI: 85.9%-98.2%), and performed well in subjects with different baseline levels of prostate-specific antigen (PSA). Even in the subgroup of subjects with very low PSA levels, PPV still exceeded 90%.

More commercially valuable, clinical data showed that more than two-thirds (67.2%) of patients were tested for TLx591-CDx, and their treatment plan was adjusted from the baseline initial plan, which fully proved the key value of this product in clinical decision-making. Yuanda Pharmaceutical plans to submit a new drug marketing application in China within this year, which is expected to provide a new standard for more accurate and efficient diagnosis of prostate cancer patients in China.
Looking at the product mechanism, TLx591-cdx and the therapeutic RDC drug TLx591 form an “integrated diagnosis and treatment” combination for prostate cancer. This strategy achieves “what you see is treatment” through the same target, greatly improving the accuracy of clinical management. Currently, TLX591 has also been approved in China to join the international multi-center phase III clinical study, and the two product combinations are ready to go.
According to our understanding, the product has five core technical characteristics: first, it can be internalized into cells to enhance signal strength; second, stable biological activity to ensure reliable detection; third, short half-life circulation in the body to reduce the patient's radiation burden; fourth, it has good penetration into the tumor substance and has a clear imaging; and finally, it can be quickly removed by non-target tissues and has a very high signal-to-noise ratio.
In overseas markets, TLx591-CDx has been approved for listing in Australia, the United States, Canada, the United Kingdom, Brazil and many European countries, showing strong international market penetration. In terms of financial performance, the product has become the company's cash cow, with global sales reaching US$517 million in 2024, while sales in the first three quarters of 2025 were about US$461 million, an increase of more than 25% over the previous year. The impressive performance not only verified the clinical value of this product, but also proved the core competitiveness of Yuanda Pharmaceutical's “integrated diagnosis and treatment” combination strategy on a global scale.
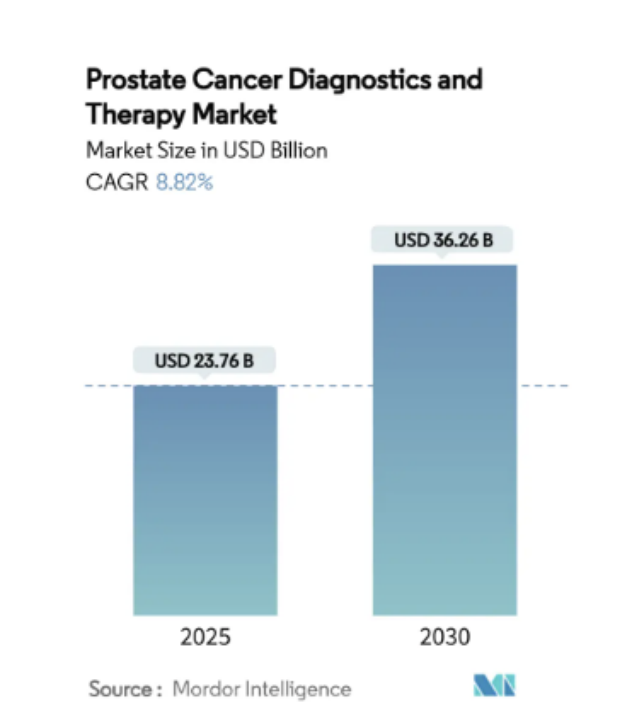
Behind this breakthrough is the vast space in the prostate cancer diagnosis and treatment market. According to Mordor Intelligence data, the global prostate cancer market is expected to grow from US$23.76 billion in 2025 to US$36.26 billion in 2030, with a compound annual growth rate of 8.82%.
Furthermore, according to Horizon research data, China's prostate cancer treatment market is expected to reach US$1,514 billion by 2030, with a compound annual growth rate of 8.5% from 2025 to 2030. Facing a blue ocean of this scale, the “integration of diagnosis and treatment” of nuclear drugs in this field has become the core racing track for giants.
It is worth mentioning that Novartis (Novartis)'s major RDC drug, Pluvicto, generated about US$1.39 billion in global revenue in 2024 and has become a benchmark in the nuclear medicine field, but China has yet to establish an absolute monopoly. This has left a key strategic window for Yuanda Pharmaceutical. With the excellent diagnostic efficacy of TLX591-CDX in phase III clinical trials in China, and the therapeutic RDC drug TLX591, which is currently undergoing international multi-center phase III clinical research, Yuanda Pharmaceutical is expected to seize market opportunities in the domestic market and build a market moat before international giants occupy the Chinese market through a closed loop strategy of “what you see, treat”.
In addition to prostate cancer, Yuanda Pharmaceutical also has an integrated diagnosis and treatment layout with multiple combinations, such as the TLx250-CDx/TLx250 combination for diagnosis and treatment of renal clear cell carcinoma (ccRCC). Among them, TLx250-CDx showed 86% sensitivity and 87% specificity in overseas phase III clinical trials, making it expected to become a new diagnostic gold standard for kidney cancer, and has already entered the US FDA's priority review channel. This multi-tumor, multi-target “integrated diagnosis and treatment” strategy has become the core competitiveness of Yuanda Pharmaceutical, which continues to lead the nuclear drug race.
GPN01530: BIC Potential and Market Prospects for FAP Targeted Nuclear Drugs
If TLx591-CDx has verified Yuanda Pharmaceutical's accurate global strategic layout capabilities, then GPN01530 successfully obtained approval from the US FDA to carry out phase I/II clinical research, and is expected to be the first self-developed product to achieve “hard core overseas”, marking a landmark step for the company in the field of independent research and development of nuclear drugs.
As Yuanda Pharmaceutical's first self-developed RDC product approved by the FDA to carry out clinical research, GPN01530 is an important milestone in the Group's global R&D and registration process. It not only reflects the company's comprehensive strength in cutting-edge nuclear drug technology platform construction, international clinical development and registration reporting, but also establishes the “China and US Double Newspaper” registration path model for the company's subsequent international development of the self-developed product pipeline.
Among them, by simultaneously carrying out research and development in the two major global core markets of China and the US, Yuanda Pharmaceutical can drastically shorten the global marketing cycle for innovative products and accelerate its occupation of the nuclear drug market.
According to the Zhitong Finance App, GPN01530's breakthrough value comes from targeting fibroblast activating protein (FAP). This target showed a very high positive expression rate in more than 90% of mainstream epithelial-derived solid tumors, including breast cancer, pancreatic cancer, and lung cancer. Because it has great potential for diagnosis and treatment of “pan-cancer types,” it is hailed by the global medical community as a “billion-dollar target for integrated diagnosis and treatment.”

(The amount of FAP expression in different tumors)
Judging from the market potential, FAP imaging agents can target the FDG PET tumor diagnosis market. Currently, China and the United States perform approximately 1.3 million FDG PET tumor imaging tests each year. Considering that FAPI drugs are up to 99% sensitive to lesion detection (significantly superior to 87% of traditional FDG) and have a higher detection rate of low FDG uptake metastases in the brain and bone, the industry estimates that it has a market volume of at least 1.5 billion yuan on the diagnostic side alone, while the global diagnostic market is expected to exceed 1 billion US dollars.
If the therapeutic side is further taken into account, the target-driven market volume is expected to expand several times or even tenfold. This not only reflects its huge commercial imagination, but also explains why global nuclear drug giants such as Novartis are investing heavily in this target direction.
In terms of diagnostic efficacy, GPN01530 shows significant best-in-class (BIC) potential: the traditional PET/CT imaging agent 18F-FDG is less sensitive in diagnosing certain cancers, about 40% to 68%, while GPN01530 optimizes the structure of FAP ligands, showing faster tumor targeting, higher tumor uptake, and better pharmacokinetic properties.
In IIT human studies that have been carried out, GPN01530 is safe, has rapid background clearance and strong and long-lasting lesion uptake. Compared with 18F-FDG, its clinical image contrast is higher and the positive lesion detection rate is more accurate.
In this context, GPN01530 is expected to become an important breakthrough in solving the problem of solid tumor diagnosis with its remarkable diagnostic advantages. As clinical research progresses, this product has great potential to reshape the solid tumor diagnosis pattern and help Yuanda Pharmaceutical seize the leading position in the global nuclear drug market.
Neffy® and GPN01360: Differentiated Innovation Breakthrough in First Aid and Chronic Disease
While deeply involved in the nuclear medicine sector, Yuanda Pharmaceutical has also achieved the gradual implementation of major products in the fields of cardiovascular and cerebrovascular first aid and the modernization of traditional Chinese medicine, and continues to broaden the company's business boundaries in various sectors through differentiated innovation strategies.
The Zhitong Finance App learned that Yuanda Pharmaceutical recently laid out exclusive commercial rights for Neffy® (Neffy®), the world's first adrenaline nasal spray for emergency treatment of type I allergic reactions, within cooperation channels in mainland China and non-exclusive commercialization rights in the Hong Kong Special Administrative Region of China.
As the first non-injectable adrenaline first aid program approved by the FDA in nearly 35 years, this product completely breaks the long-standing dependency on injectable administration, enabling patients to quickly complete self-help when severe anaphylactic shock occurs. Clinical study data prove that Neffy® is significantly superior to standard intramuscular injections in improving core pharmacodynamic indicators such as pulse rate and systolic blood pressure, and its 30-month shelf life effectively reduces patients' reserve burden.
According to Frost & Sullivan data, China's allergic disease drug market is expected to reach 9.6 billion US dollars in 2025 and 16.2 billion US dollars in 2030, of which the adrenaline market will reach 1.1 billion US dollars in 2025. Among them, the 2mg specification of Neffy® has now been approved for listing in mainstream global markets such as the US, Europe, and Japan. Its sales have shown an exponential growth trend, and its commercial value has been fully verified by the market.
With the acceptance of the NDA application for this product in China, Yuanda Pharmaceutical is expected to promote the rapid penetration of emergency medicine into various out-of-hospital scenarios such as homes, schools, and travel through its unique portability and ease of operation, to fill the gap in out-of-hospital intervention for severe allergic reactions in China, and plans to achieve localized production within 24 months after approval, further consolidating the company's leading position in the field of first aid.
Furthermore, Yuanda Pharmaceutical's exploration in the fields of modernization of traditional Chinese medicine and neurology has also made substantial breakthroughs. Its self-developed Class 1.1 innovative traditional Chinese medicine drug GPN01360 performed well in domestic phase II clinical studies on depression, successfully reached the main clinical endpoints, and showed significant efficacy and safety advantages.
Currently, common pain points such as delayed efficacy and large side effects of antidepressants are common. GPN01360 is based on the ancient classic prescription “Xiaoyao San” to reveal the innovative mechanism of traditional Chinese medicine in regulating gut microbes and metabolic pathways through scientific means. It not only provides patients with depression with more curative efficacy and safety advantages, but also marks that Yuanda Pharmaceutical has successfully expanded its traditional advantages in the field of traditional Chinese medicine from facial medicine to a more promising neurology circuit.
Currently, the market for depression drugs in China continues to expand as public health awareness increases. According to Insight Consulting, the market is expected to grow at a compound annual growth rate of 8.1% to 10 billion by 2029. The clinical success of GPN01360 not only filled the market demand for high-safety anti-depressant traditional Chinese medicines, but also reflected Yuanda Pharmaceutical's firm determination on the path of “modernization of traditional Chinese medicine”.

Through collaborative efforts and landmark breakthroughs in various core sectors such as nuclear drug anti-tumor diagnosis and cerebrovascular first aid, Yuanda Pharmaceutical is steadily building a comprehensive innovation map across treatment fields. While providing more high-quality treatment options for patients around the world, it continues to promote the long-term investment value of the company.
This innovative breakthrough across fields is no accident; it is a systematic result of Yuanda Pharmaceutical's long-term adherence to the “independent development+global expansion” strategy. Through deep cultivation of differentiated racetracks with multiple advantages, the company has formed a comprehensive innovation pattern with multiple blossoming and balanced development.
Up to now, the company has built 8 R&D centers and 5 core technology platforms around the world. The total number of ongoing research projects has reached 133, including 42 innovative projects with great clinical potential. Furthermore, the company has always maintained a high level of investment in cutting-edge research, investing more than HK$1 billion in the first half of 2025.
Through continuous capital inclination and resource integration, Yuanda Pharmaceutical has secured the stepwise implementation of innovation pipelines in various sectors, and with its unique “integrated diagnosis and treatment” concept and closed loop throughout the industry chain, the nuclear medicine sector has taken the lead in becoming a benchmark for the company's high-quality development results transformation, and is also the core growth engine with extremely explosive and competitive barriers in the company's “Go Global” strategy.
Closed loop deep empowerment of the entire nuclear medicine industry chain: the “Go Global” strategy has entered a period of implementation of results, driving future high-quality growth
Whether it is the successful commercialization of TLx591-CDx overseas or the “hard core overseas” of the GPN01530 self-developed project, its deep roots stem from the closed loop of a global and comprehensive industrial chain built by Yuanda Pharmaceutical in the field of nuclear drug anti-tumor diagnosis and treatment.
At present, Yuanda Pharmaceutical has achieved a comprehensive layout of nuclear drugs from early R&D, clinical research, registration and registration to production, sales, and regulatory qualifications, and has become one of only four pharmaceutical companies in the world that have successfully commercialized innovative nuclear drugs globally.
The Zhitong Finance App learned that up to now, around the concept of “integrated diagnosis and treatment”, Yuanda Pharmaceutical has deep reserves of 16 innovative global products during the R&D and registration stage, covering 5 types of radionuclides, including 68 Ga, 177Lu, 131I, 90Y, and 89Zr, covering 7 major types of cancer, including liver cancer, prostate cancer, brain cancer, and gastrointestinal pancreatic neuroendocrine tumors. It is currently the company with the largest reserves of RDC innovative drugs in phase III clinical research in China, forming an extremely stable first-mover advantage and competitive barrier.
Today, the advantages of this entire industry chain layout are rapidly being transformed into strong commercialization results, indicating that Yuanda Pharmaceutical's nuclear medicine sector has officially entered the value realization period after years of intensive cultivation.
Take Yuanda Pharmaceutical's core product, Yigantai® Yttrium [90Y] microsphere injection, as an example. The product has shown explosive growth since it was approved for marketing in China. In 2024, sales revenue reached nearly HK$500 million, a year-on-year growth rate of over 140%, and continued to maintain a strong trend of doubling revenue in the first half of 2025. Thanks to its excellent clinical efficacy and increasingly improved admissions access, the product is expected to reach a commercialization milestone of HK$1 billion this year.
Looking at market space, the global radiopharmaceuticals market is expected to rise to 57.3 billion US dollars by 2035, with a compound annual growth rate of 17.5%. The Chinese market is expected to reach 75.8 billion yuan during the same period. In this vast blue ocean circuit, the company is steadily unleashing the strategic dividends it has accumulated over a long period of time with a comprehensive industrial layout.
After years of forward-looking layout and advanced technology development, Yuanda Pharmaceutical has a leading edge in the global innovation and development of “Go Global”, represented by the nuclear medicine sector, and has formed a global R&D, production and marketing integrated system composed of R&D bases centered in Boston and Chengdu, as well as production bases in Boston, Frankfurt, Singapore, and Chengdu, and sales networks covering more than 50 countries and regions around the world.
At the same time, with the official commissioning of the Chengdu Nuclear Drug R&D and Production Base, the world's first closed-loop platform for the entire nuclear drug industry chain, Yuanda Pharmaceutical achieved 100% independent production of isotope preparation and nuclear drug formulations through 14 high-standard GMP production lines, completely solved the “stuck neck” problem in nuclear drug raw materials and production processes, and truly achieved complete autonomy and control of the entire R&D, production and sales industry chain of innovative nuclear drug products, providing a solid guarantee for the localization and large-scale commercialization of the company's nuclear drug products.
Looking forward to the future, with the successful development and registration of more innovative products on a global scale, such as the US listing application for ITM-11 being officially accepted by the FDA and the orderly progress of the TLx591-CDx application for listing in China, Yuanda Pharmaceutical is expected to further unleash the synergetic value of self-developed and imported assets, achieve continuous strong growth, and continue to consolidate its leading position in the global nuclear medicine field.